Energy in the form of ATP can be generated from glucose without oxygen in two ways, fermentation and anaerobic metabolism. Both occur when oxygen-using (aerobic) cellular metabolism is lacking because of insufficient oxygen to accept the electron at the end of the electron transport chain. Some biochemists refer to the conversion of pyruvate to lactate as fermentation but this is confusing terminology. To most people, fermentation refers to the production of ethanol not lactic acid.
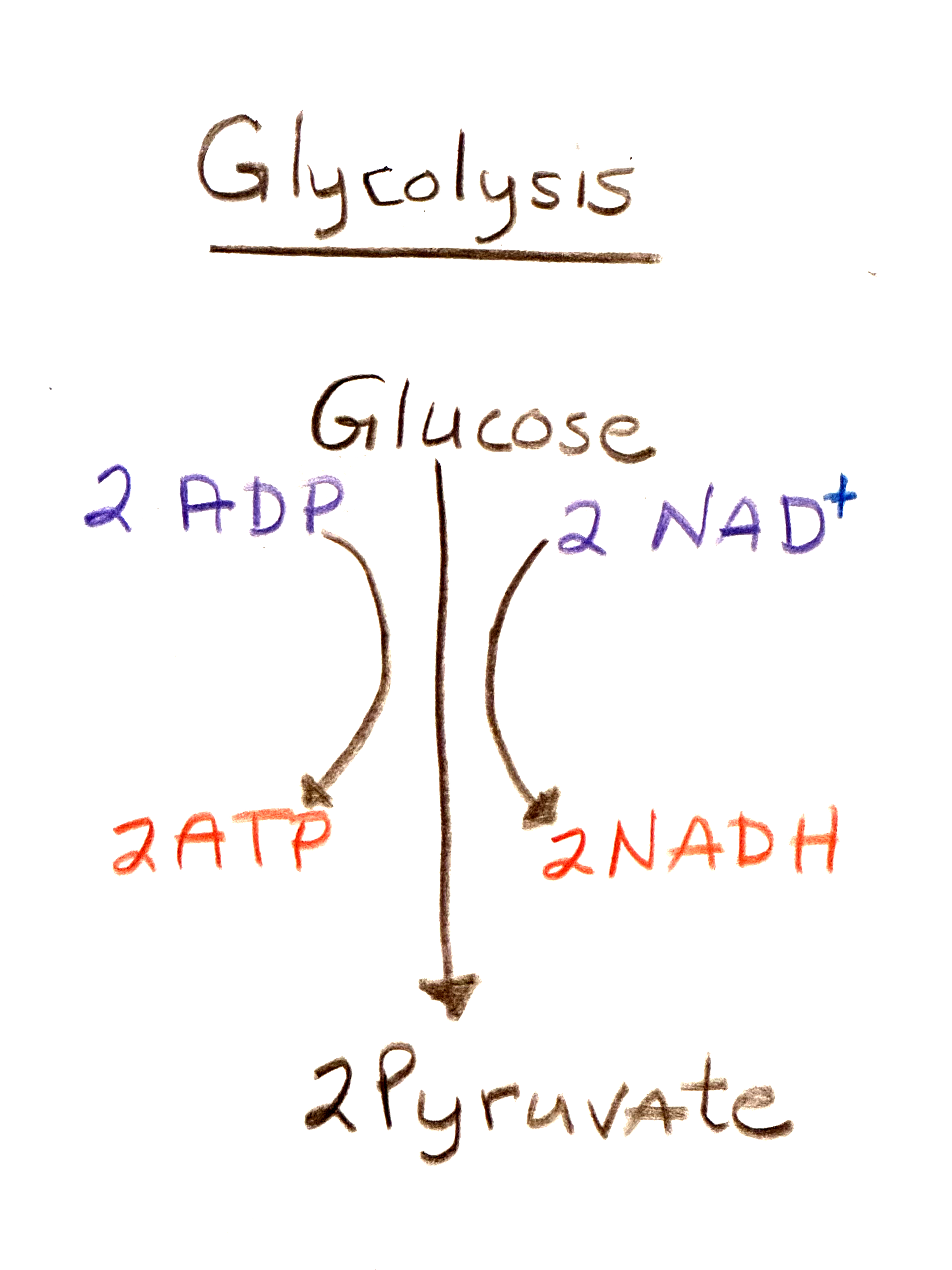
The first part in either case is glycolysis in a series of steps that results in the production of pyruvate. One glucose molecule is converted to two pyruvate molecules, producing two net ATP and two NADH. This is an energy producing process as it produces 2 ATP.

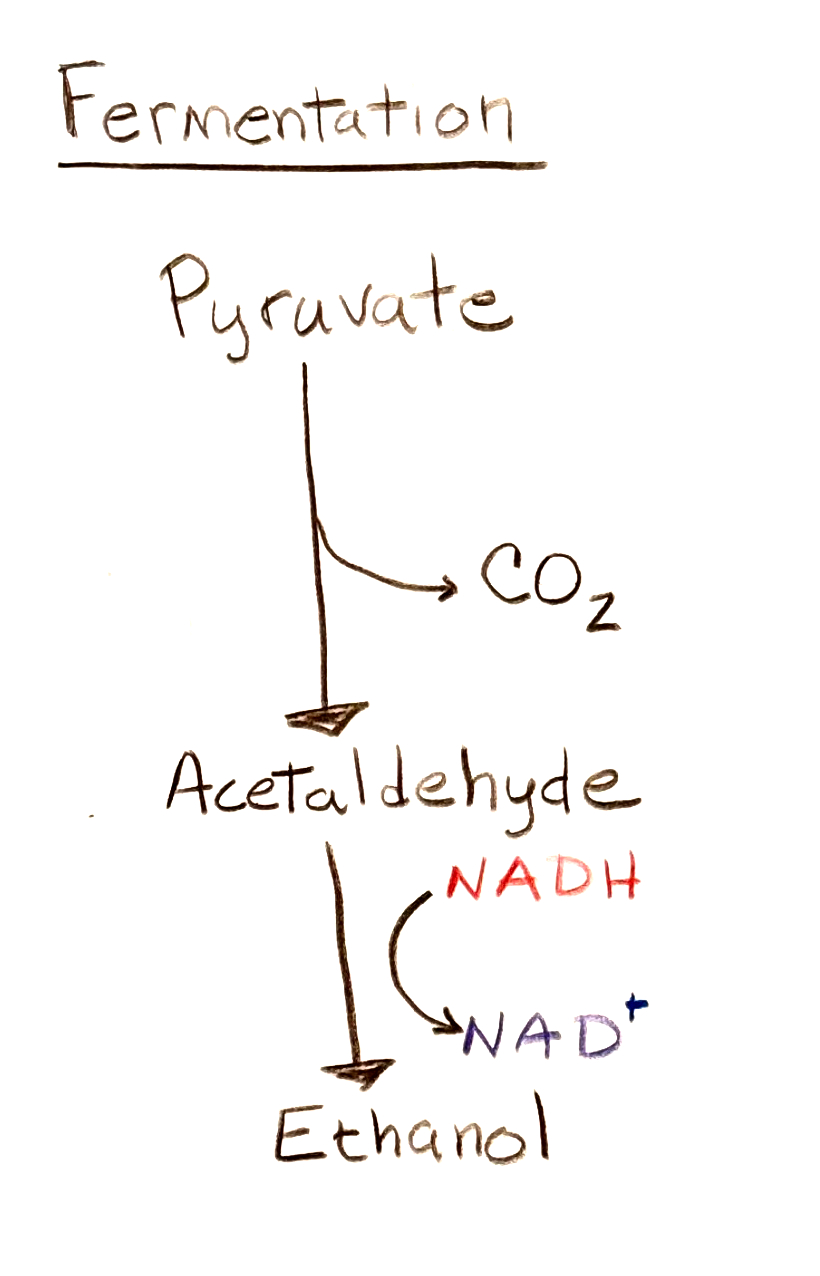
In fermentation a carboxyl group is removed from pyruvate and released as carbon dioxide, producing acetaldehyde. The CO2 generated can be retained to produce beer and sparkling wines. This is a process used by yeast and other microorganisms. The acetaldehyde is then converted to ethanol. Fermentation produces alcohol while anaerobic metabolism produces lactic acid.
The two NADH donate electrons and hydrogen atoms to the two acetaldehyde molecules, producing two ethanol molecules. This regenerates the electron carrier NAD+ from the NADH produced in the first step, glycolysis.
 It is important that there is
no lactate or any other anionic substance without a cation such as a
hydrogen ion, also known as H+ or a proton. Sometimes the proton, H+
is omitted from these equations but anaerobic metabolism produces
protons contributing to acidemia as well as the conjugate base
lactate. There is no question that anaerobic metabolism decreases pH.
It also produces increased concentrations of lactate and lactic acid.
The ratio of lactate present as lactate in combination with inert
cations such as sodium, hence a base and lactic acid meaning lactate
combined with a proton, hence an acid increases. This combination is
measured and reported to doctors as lactate but it is mostly lactic
acid.
It is important that there is
no lactate or any other anionic substance without a cation such as a
hydrogen ion, also known as H+ or a proton. Sometimes the proton, H+
is omitted from these equations but anaerobic metabolism produces
protons contributing to acidemia as well as the conjugate base
lactate. There is no question that anaerobic metabolism decreases pH.
It also produces increased concentrations of lactate and lactic acid.
The ratio of lactate present as lactate in combination with inert
cations such as sodium, hence a base and lactic acid meaning lactate
combined with a proton, hence an acid increases. This combination is
measured and reported to doctors as lactate but it is mostly lactic
acid.
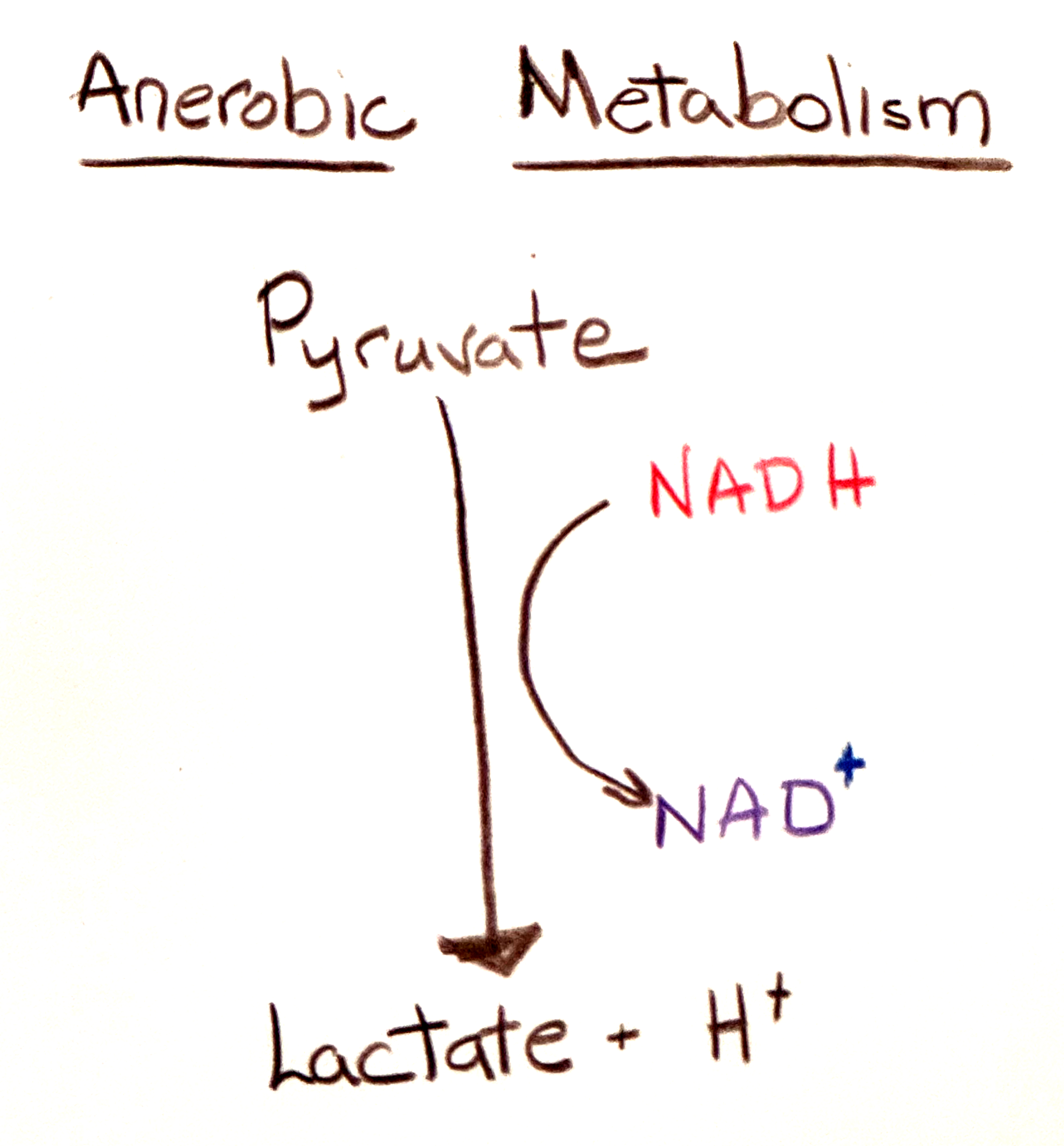
In the second part of anaerobic metabolism, NADH donates electrons and hydrogen atoms to pyruvate molecules, producing lactate molecules and regenerating NAD+.
Lactic acid is lactate along with a proton which is easily dissociated from the conjugate base lactate. The process of anaerobic metabolism produces not only the lactate but also the proton. The concentration of lactate increases, a measure of the rate of anaerobic metabolism but it is the proton that accompanies the lactate that decreases pH.
Anaerobic metabolism occurs to a certain extent under normal circumstances notably in the red blood cells, which don’t have mitochondria and thus can’t perform aerobic cellular respiration. Muscle cells also carry out anaerobic metabolism, particularly when they have too little oxygen for aerobic respiration to fully supply the metabolic requirements. Lactic acid buildup is associated with exercise particularly middle distance, 800 and 1600 meter races. These are intense enough and long enough to produce significant acidemia (reduced pH) due to the production of lactic acid. The resulting decrease in pH is moderated by the increased respiratory rate and depth of respiration that invariably can be observed in individuals at the end of such races.
Most of the lactic acid produced in muscle cells is transported through the bloodstream to the liver, where it’s converted back to pyruvate. This requires oxygen but will replace the oxygen debt incurred by the production of lactic acid.
It was once asserted that the accumulation of lactate in muscles was responsible for the soreness caused by exercise. There was never any good evidence that this was the case. Lactate levels do increase in highly metabolic tissues with strenuous exercise. PH also decreases along with the accumulation of lactate. The increase in lactate in the extremities is accompanied by muscle soreness. Associations do not prove causality. It is not possible to know through any ethical or practical experiment that the associated lactate is the cause of muscle soreness. The pH is also a factor. The prevailing opinion in the past was was that lactate caused of muscle soreness. The pendulum of consensus has swung the other way with the more recent general consensus is that lactate is not the cause of muscle soreness. PH is commonly omitted from the analysis of these experiments. The proper experiment has not been done and as a practical matter never will be.